Charles Zhao, M.D., MBA, brings more than 20 years of drug development experience in oncology therapeutic area. Before Elpis, Charles served as CMO at ABM Therapeutics and Sr. Medical Director at Mirati Therapeutics. Previously, he was Medical Director at EMD Serono working on cMET inhibitor global program in hepatocellular carcinoma indication; MEK inhibitor early phase development program in multiple indications including pancreatic cancer, ovarian cancer, melanoma, NSCLC, CRC, TNBC, and CML; and MetAP2 inhibitor early phase development program. He was Associate Medical Director at Boehringer Ingelheim working on PLK1 inhibitor in urothelial carcinoma and AML indication. His career highlights also include his work at Novartis working on HDAC inhibitor in CTCL and PKC inhibitor in AML indication, and Eli Lilly in early phase clinical development.
Charles Zhao, M.D., MBA, brings more than 20 years of drug development experience in oncology therapeutic area. Before Elpis, Charles served as CMO at ABM Therapeutics and Sr. Medical Director at Mirati Therapeutics. Previously, he was Medical Director at EMD Serono working on cMET inhibitor global program in hepatocellular carcinoma indication; MEK inhibitor early phase development program in multiple indications including pancreatic cancer, ovarian cancer, melanoma, NSCLC, CRC, TNBC, and CML; and MetAP2 inhibitor early phase development program. He was Associate Medical Director at Boehringer Ingelheim working on PLK1 inhibitor in urothelial carcinoma and AML indication. His career highlights also include his work at Novartis working on HDAC inhibitor in CTCL and PKC inhibitor in AML indication, and Eli Lilly in early phase clinical development. Diala Ezzeddine, Ph.D. Diala is founder and CEO of Xios Therapeutics (Waltham, MA), a privately held, venture-backed drug discovery company. Xios is developing next generation oncology protein degrading therapeutics with the potential to transform the treatment and lives of cancer patients. From 2010 to 2015, Diala was EVP and Chief Business Officer of X-Chem. She was instrumental in crafting the company’s business strategy, obtaining an equity investment, and establishing multiple revenue generating partnerships with top tier pharmaceutical companies with a cumulative deal value surpassing $2B. In the course of her career, Diala held business development roles of increasing responsibility at several leading biotechnology companies in the Boston area including Millennium Pharmaceuticals (Cambridge, MA, now Takeda Pharmaceuticals), and Archemix Corp. (Cambridge, MA). In support of the companies’ corporate strategies, she structured and negotiated value creating partnerships with several of the world’s largest pharmaceutical companies. Diala holds a Ph.D. in Genetics from Harvard Medical School obtained in 1997. Before that, she had conducted research in the Neurogenetics department at Massachusetts General Hospital (Boston, MA). She is the lead author on a publication describing successful in vivo pre-clinical proof-of-concept for an innovative genetic engineering approach to treating malignant brain tumors.
Diala Ezzeddine, Ph.D. Diala is founder and CEO of Xios Therapeutics (Waltham, MA), a privately held, venture-backed drug discovery company. Xios is developing next generation oncology protein degrading therapeutics with the potential to transform the treatment and lives of cancer patients. From 2010 to 2015, Diala was EVP and Chief Business Officer of X-Chem. She was instrumental in crafting the company’s business strategy, obtaining an equity investment, and establishing multiple revenue generating partnerships with top tier pharmaceutical companies with a cumulative deal value surpassing $2B. In the course of her career, Diala held business development roles of increasing responsibility at several leading biotechnology companies in the Boston area including Millennium Pharmaceuticals (Cambridge, MA, now Takeda Pharmaceuticals), and Archemix Corp. (Cambridge, MA). In support of the companies’ corporate strategies, she structured and negotiated value creating partnerships with several of the world’s largest pharmaceutical companies. Diala holds a Ph.D. in Genetics from Harvard Medical School obtained in 1997. Before that, she had conducted research in the Neurogenetics department at Massachusetts General Hospital (Boston, MA). She is the lead author on a publication describing successful in vivo pre-clinical proof-of-concept for an innovative genetic engineering approach to treating malignant brain tumors.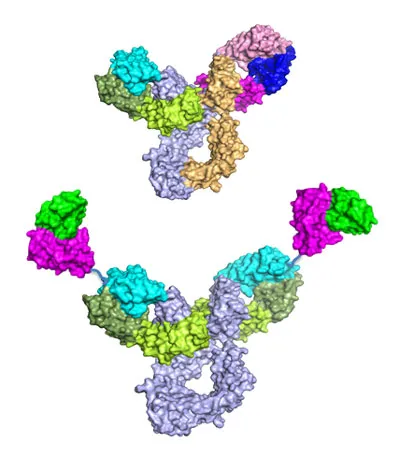
Cancer is a heterogeneous disease whose pathogenesis is often driven by multiple signaling pathways or genetic alterations which evolve with treatment and metastasis. We are developing bispecific antibodies to overcome this therapeutic challenge. Bispecific antibodies can simultaneously recognize multiple tumor surface antigens and/or active immune cells. The potential treatment outcomes of the bispecific antibodies may be superior to that associated with existing treatments which address just a single pathway or genetic alteration. Elpis is developing bispecific antibodies against tumor specific antigens, pathologic pathways and tumor microenvironment targets.
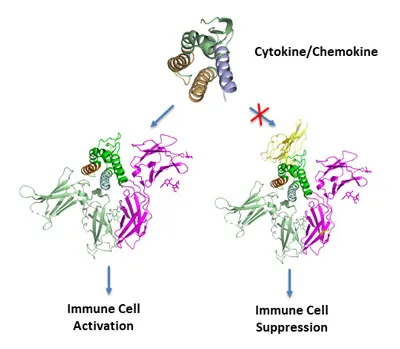
Activation of certain immune cells, such as NK cells and cytotoxic T-Cells, can effectively eradicate tumor lesions. However, immunosuppressive cells including regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages may counteract this immune activation, particularly in the tumor microenvironment. Cytokines produced by immune cells, such as IL-2, TNF-a, and IL-10, are important for the regulation of immune function. Utilizing our mSCAFoldTM technology, we can deliberately engineer biased cytokines or chemokines to stimulate immune cells to fight cancer, while downregulating suppressive immune cells. Such rational design-based immuno-modulators may lead to more effective new therapies with less systemic toxicity. This technology can also redirect functions of other important proteins positioning for better therapeutic efficacies. EPIM-001 is the leading immuno-modulator drug candidate for various solid tumors. We are conducting animal studies to understand its mechanism of action and tumor killing efficacy. We plan to advance EPIM-001 to clinical studies in early 2021.
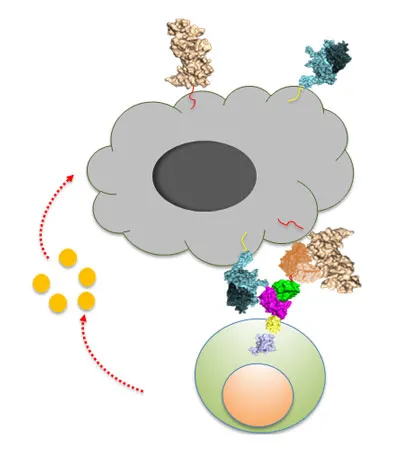
Adoptive cell therapy is a rapidly emerging immunotherapeutic in which a patient’s own immune cells are engineered to fight against their cancer cells. CAR (Chimeric Antigen Receptor)-T cells are T-cells engineered with a tumor specific antibody and T cell signaling domains to enable a specific engagement of immune cells to the cancer cells. While CAR T cells have demonstrated incredible clinical results thus far, patients often relapse due to tumor antigen loss. To combat tumor antigen loss and subsequent tumor escape, we are developing next generation CAR-T cell therapeutics that target two or more tumor specific/associated antigens simultaneously. This may significantly improve the therapeutic response and duration of CAR-T treatment. Elpis is currently developing a dual targeting CAR-T drug candidate EPC-001 for hematologic tumors. We plan to advance the drug to phase I clinical trial in early 2021.
 Ahmad is the Vice President of Business Development and Corporate Strategy. Previously, he held roles in business development and corporate strategy at Epizyme and Momenta Pharmaceuticals. His diverse drug development experience includes roles in management consulting, commercial strategy, corporate finance, and program and portfolio management. Ahmad started his career in drug discovery as a research scientist at Abbvie, Vertex and Chiron. He holds a B.S in Chemistry from University of Massachusetts, an M.S. in Pharmacology from Northeastern University, and an MBA from F.W. Olin College of Business at Babson College.
Ahmad is the Vice President of Business Development and Corporate Strategy. Previously, he held roles in business development and corporate strategy at Epizyme and Momenta Pharmaceuticals. His diverse drug development experience includes roles in management consulting, commercial strategy, corporate finance, and program and portfolio management. Ahmad started his career in drug discovery as a research scientist at Abbvie, Vertex and Chiron. He holds a B.S in Chemistry from University of Massachusetts, an M.S. in Pharmacology from Northeastern University, and an MBA from F.W. Olin College of Business at Babson College. Yan Chen, PhD: Founder, President and CEO. Yan brings more than 20 years of expertise in both mRNA display technology and biologic drug discovery in the oncology disease area. She was Biotherapeutics Discovery Head at Juno Therapeutics in Boston and Senior Vice President of Research at X-Body Biosciences which was acquired by Juno in 2015. Previously, she was an investigator and project leader in Oncology Biotherapeutics at Novartis Institutes for Biomedical Research. Earlier in her career, she was a scientist at Phylos and Adnexus playing instrumental roles in establishing the mRNA antibody and protein display technology platforms. Yan was a postdoctoral trainee in Immunology at Tufts Medical School and received her PhD in Tumor Immunology from National University of Singapore. She is the co-inventor of more than 20 antibody technologies and biologic therapeutics patents.
Yan Chen, PhD: Founder, President and CEO. Yan brings more than 20 years of expertise in both mRNA display technology and biologic drug discovery in the oncology disease area. She was Biotherapeutics Discovery Head at Juno Therapeutics in Boston and Senior Vice President of Research at X-Body Biosciences which was acquired by Juno in 2015. Previously, she was an investigator and project leader in Oncology Biotherapeutics at Novartis Institutes for Biomedical Research. Earlier in her career, she was a scientist at Phylos and Adnexus playing instrumental roles in establishing the mRNA antibody and protein display technology platforms. Yan was a postdoctoral trainee in Immunology at Tufts Medical School and received her PhD in Tumor Immunology from National University of Singapore. She is the co-inventor of more than 20 antibody technologies and biologic therapeutics patents. Kehao Zhao, PhD: Co-founder, Chief Scientific Officer. Kehao brings more than 15 years of drug discovery experience in disease areas including cancer, liver disease and ophthalmology. Before Elpis, Kehao was a senior investigator at Novartis Institutes for Biomedical Research leading antibody therapeutics platform and cancer epigenetics research. Previously, Kehao was a scientist at Procter & Gamble Pharmaceuticals. Kehao completed postdoctoral training in Gene Expression & Regulation at the Wistar institute and received his PhD from the Institute of Biophysics at the Chinese Academy of Sciences. Kehao has published over 30 research papers in prestigious scientific journals.
Kehao Zhao, PhD: Co-founder, Chief Scientific Officer. Kehao brings more than 15 years of drug discovery experience in disease areas including cancer, liver disease and ophthalmology. Before Elpis, Kehao was a senior investigator at Novartis Institutes for Biomedical Research leading antibody therapeutics platform and cancer epigenetics research. Previously, Kehao was a scientist at Procter & Gamble Pharmaceuticals. Kehao completed postdoctoral training in Gene Expression & Regulation at the Wistar institute and received his PhD from the Institute of Biophysics at the Chinese Academy of Sciences. Kehao has published over 30 research papers in prestigious scientific journals.